
this is two positive charges and one negative charge.If the formula of an ionic compound needs more than one polyatomic ion, the formula of this ion is written inside brackets.Ĭalcium hydroxide contains Ca 2+ and OH - ions:
Polyatomic ions are formed from groups of two or more atoms. to make the number of charges the same, we need two Al 3+ ions and three O 2- ions.this is three positive charges and two negative charges.this is two positive charges and two negative chargesĪluminium oxide contains Al 3+ and O 2- ions:.Magnesium oxide contains Mg2 + and O2 - ions: the number of charges are already the same.
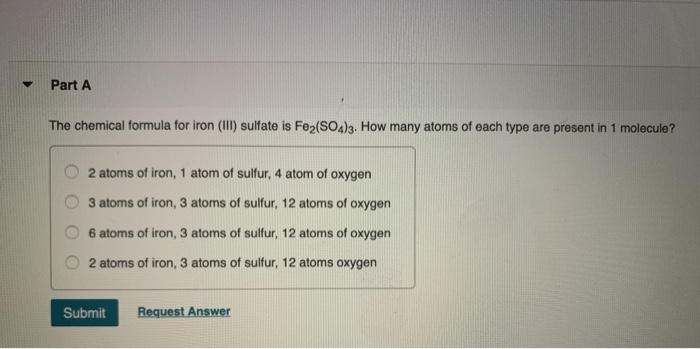
CHEMICAL FORMULA FOR IRON CHARGE HOW TO
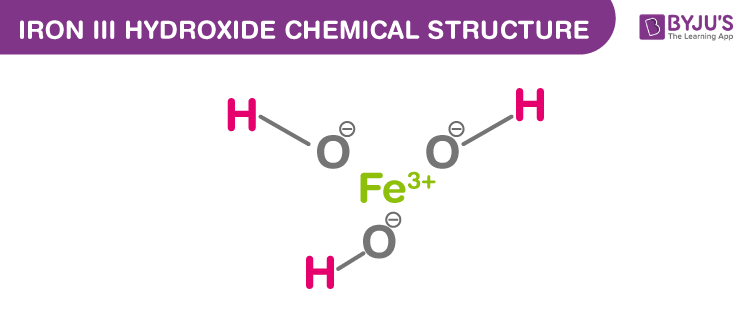
this is one positive charge and one negative charge How to write the formula:- The central metal ion is iron (III) ion, which has a charge of +3, and is represented by Fe3+.- The hexacyanoferrate (II) ion is.Sodium chloride contains Na + and Cl - ions: The formula for an ionic compound must give the same number of positive and negative charges. Positive ionsĪlthough ionic compounds contain electrically charged ions, they are neutral overall. If your research funder has signed Plan S, your open access charges may be covered by your funder through December 31, 2024. Similarly, the non-metal elements in groups 6 (IUPAC group 16) and 7 (IUPAC group 17), the ionic charge can be deduced by working out how many electrons must be gained to fill the outer shell. Remember that for the metal elements in groups 1, 2, and 3 the charge on the ion can be deduced by how many outer shell electrons there were in the neutral atom. The formula of an ionic compound can be deduced from the formulae of its ions. They almost always contain at least one metal element and at least one non-metal element. Step 3 Combine ions so that the charges cancel.Ionic compounds are made up of oppositely charged ions joined together by ionic bonds. Step 2 Determine the charge of the anion. The name of a Type II cation contains the charge! iron (III) means Fe 3+. Step 1 determine the charge of the cation. Explanation: Math Processing Error iron Math Processing Error nitrogen Since its Math Processing Error, Math Processing Error must have a Math Processing Error charge to match the Math Processing Error charge on Math Processing Error in order. The charges of the cations and the anions must be known to determine the formula of the compound. Thus, the nomenclature rules for ionic compounds are used. Iron is a metal and oxygen (oxide) is a nometal therefore, iron(III) oxid is an ionic compound. Three protons are needed to cancel the charge on the phosphate ion, PO 4 3-, so the formula is H 3PO 4.įor example, iron (III) oxide.
The name doesn't use the hydro something acid notation, so the phosphoric must be referring to the polyatomic ion phosphate. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Thus, the acid nomenclature rules are used. Element Iron (Fe), Group 8, Atomic Number 26, d-block, Mass 55.845. The name has the word acid in it therefore, it is an acid. One proton is needed to cancel the charge of the chloride ion, so hydrochloric acid is HCl.Įxample, phosphoric acid. The chloro is referring to the chloride anion, Cl. Only acids based on monoatomic ions use the hydro something acid notation. Thus, the acid nomenclature rules are used. Thenumbersat thetopdenote the chemical formula of the compound underneath (for instance. The name has the word acid in it therefore, it is an acid. Fig.2.1 aSeveral representative classes of the iron-based materials. ( hint: For our purposes, only covalent compounds use prefixes therefore, the presence of a prefix means the compound must be covalent.)Īcids: add as many protons (H +) as needed to cancel the charge of the anion.įor example, hydrochloric acid. Thus, the covalent molecule nomenclature rules are used.Ĭarbon is C, idodide is I, and tetra means 4, so carbon tetraiodide is CI 4. Carbon is a nonmetal, and iodide is a nonmetal therefore, carbon and iodide form a covalent compound. The anion may or may not be polyatomic.Ĭovalent compunds: write the element with a subscript that corresponds to the prefix.įor example, carbon tetraiodide. Covalent compounds are made from two nonmetals. Ionic compounds are made from a metal and a nonmetal. To determine a formula of a compound from its name you have to identify a compound as Ionic Compound Since the magnitude of the charges are the same, the ions will combine in a 1:1 ratio, and the formula for magnesium oxide is MgO.


 0 kommentar(er)
0 kommentar(er)
