

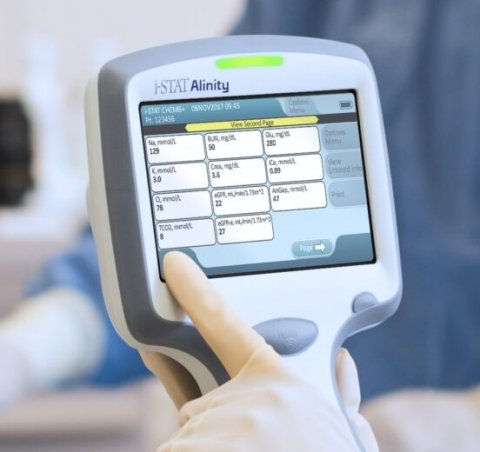
Xtant Medical announces $15M private placement.Study backs Lunit’s AI-powered cancer diagnostics.Yesterday, Abbott said it won CE Mark approval in the European Union for its next-generation drug-eluting stent, the Xience Sierra.įiled Under: 510(k), Diagnostics, Food & Drug Administration (FDA), Regulatory/Compliance Tagged With: Abbott The company said that in addition to the instrument clearance, it has also obtained clearance for several tests on the system with a “comprehensive menu of tests expected to be available within a year of launch,” according to a press release. With customer insights as our roadmap, the Alinity ci is engineered to simplify diagnostic testing while ensuring speed, accuracy and performance,” immunoassay and clinical chemistry R&D VP John Frels said in prepared remarks. We went beyond traditional market research and spent countless hours with our customers, listening to their challenges and observing how they work. “Alinity ci was designed using a different approach.
#Abbott istat fda approval full#
customers as we work to gain approval for the full Alinity portfolio of instruments and assays,” Abbott diagnostic products exec VP Brian Blaser said in a prepared statement.Ībbott said its Alinity system includes the Alinity c clinical chemistry system and the Alinity i immunoassay system, both of which can operate individually or work together as an integrated Alinity ci-series unit. FDA clearance is a key first step in bringing this important innovation to our U.S. Labs and healthcare systems are looking for complete solutions that help them operate more efficiently while contributing to better clinical decision making and helping improve patient outcomes. “Healthcare systems across the United States are under pressure to deliver better care for patients. The newly launched ci-series of Alinity devices includes improvements such as smaller footprints, improved workflow capabilities and reduced wait times and simplified designs for error proofing and enhanced usability, the company said. While some point-of-care tests are approved for a CLIA waiver, advances in technology that enhance the rapidity of testing enable more complex, nonwaived testing to be performed at or near the site of patient care.Abbott (NYSE: ABT) said today it won FDA 510(k) clearance for its Alinity instruments for clinical chemistry and immunoassay diagnostics.
“ FDA-cleared external icon ” means a test system has been reviewed by the FDA and has been determined to be substantially equivalent to a test system already legally marketed for the same use.“CLIA-exempt” formally refers to a laboratory (not a test system) and means a laboratory that has been licensed or approved by a state where CMS has determined that the state has enacted laws relating to laboratory requirements that are equal to or more stringent than CLIA requirements, and the State licensure program has been approved by CMS (42 CFR 493.2).These terms do not relate directly to the test complexity categories, and it cannot be assumed that a test system is waived simply because it is performed at the point of care.
Several terms including “CLIA-exempt,” “FDA-cleared,” and “point-of-care testing” might mistakenly be confused with CLIA-waived testing.


 0 kommentar(er)
0 kommentar(er)
